 In 1747, James Lind proved that scurvy could be prevented by a diet that included citrus fruit. It wasn’t until 1912 that the active ingredient, ascorbic acid (later called vitamin C), was discovered. Scurvy has been relatively rare since that time in populations with access to a varied diet. We likely wouldn’t have pharmacy shelves lined with bottles of vitamin C today if it were not for Linus Pauling, who spent the later parts of an otherwise brilliant scientific career touting megavitamin therapy, with, among other supplements, vitamin C. Since the 1970s, massive doses of vitamin C in those without deficiency have shown no meaningful medicinal effects, but the vitamin retains a health halo among alternative medicine proponents that is utterly resistant to research that shows it to be, on balance, useless.
In 1747, James Lind proved that scurvy could be prevented by a diet that included citrus fruit. It wasn’t until 1912 that the active ingredient, ascorbic acid (later called vitamin C), was discovered. Scurvy has been relatively rare since that time in populations with access to a varied diet. We likely wouldn’t have pharmacy shelves lined with bottles of vitamin C today if it were not for Linus Pauling, who spent the later parts of an otherwise brilliant scientific career touting megavitamin therapy, with, among other supplements, vitamin C. Since the 1970s, massive doses of vitamin C in those without deficiency have shown no meaningful medicinal effects, but the vitamin retains a health halo among alternative medicine proponents that is utterly resistant to research that shows it to be, on balance, useless.
COVID-19 emerged just over a year ago and has resulted in a pandemic the likes of which has not been seen in generations. While many patients only experience mild symptoms, some patients progress to severe illness, hospitalization, and death. Because most cases are mild and because the likelihood of progressing to severe disease isn’t perfectly predictable, it is perhaps not surprising that (1) many people, understandably fearing severe disease, will try any number of folk remedies, and (2) because most people recover, that their recovery may be attributed to whatever treatment approach they followed, no matter how unorthodox or implausible.
Vitamin C and zinc have been touted as effective treatments for the common cold for decades. While the evidence is poor for vitamin C and weak (but better) for zinc, the virus that causes COVID-19, SARS-CoV-2, is a coronavirus, and coronaviruses can also cause the common cold. Yes it’s a stretch, but anything that could potentially help reduce the morbidity and mortality of COVID-19 would be welcomed, and given the public’s embrace of zinc and vitamin C for colds, it was perhaps warranted to test these two products for their effectiveness against COVID-19 disease. The results will not surprise you.
The evidence to date
I blogged about zinc and COVID-19 last November. At that time there were no clinical trials that studied zinc for preventing or treating SARS-CoV-2 infections. The evidence for colds (which can be caused by coronaviruses) is promising, but weak overall, only possibly modestly reducing the duration of colds. In terms of COVID-19 trials with Vitamin C, I found 45 clinical trials in the COVID-19 / WHO clinical trials database. Searching PubMed for published clinical trials, I could only find this protocol studying intravenous high-dose vitamin C, and the results have not been published. Another open-label trial found no benefit from high-dose intravenous vitamin C. An open-label pilot trial in China also showed no benefit in terms of “invasive mechanical ventilation-free days”. Even outside of COVID-19 infections and looking at the critically ill more generally, there is no strong evidence for its use. So that brings us to the current trial.
COVID A to Z
This trial was published on February 12, 2021, and is from Thomas et al. from the Cleveland Clinic. The “COVID A to Z Clinical Trial” was a randomized controlled trial intended to study adults diagnosed with a SARS-CoV-2 infection who were expected to recover at home. The trial enrolled only adults and participants were randomized to one of four groups:
- 8,000 mg of ascorbic acid (to be divided over 2-3 times per day with meals) for 10 days,
- 50 mg of zinc gluconate at bedtime for 10 days,
- both therapies for 10 days, or
- usual care (no treatment)
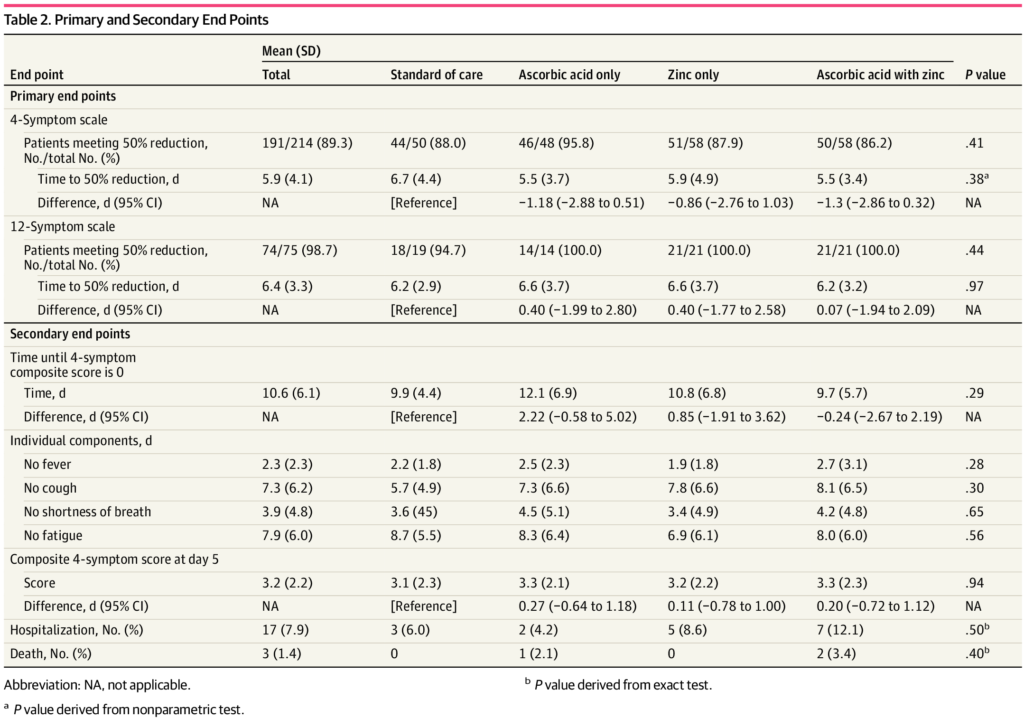
Participants were asked to track their symptoms daily, and to complete a survey until day 28 to assess hospitalization and adverse effects. The primary end point was the number of days from the time of peak symptom score to a 50% resolution in those symptoms. They also measured the number of days to reach a symptom score of zero, as well as the incidence of hospitalizations, death, other medications, and adverse events.
The trial was intended to recruit 520 participants but an interim analysis was completed after 214 patients were enrolled. Based on pre-specified stopping rules, the trial was then terminated for futility, as there were no meaningful differences between the three treatment groups and the control group that received no treatment.
The average age of participants was 45 years old, 62% women and 38% were current or former smokers. At least one-quarter had used vitamins and minerals previously.
There was no significant difference in the time required to reach a 50% reduction in symptoms. In the usual care group, this was 6.7 days, while it was 5.5 days in the ascorbic acid group, 5.9 days in the zinc gluconate group, and 5.5 days in the group that received both products:
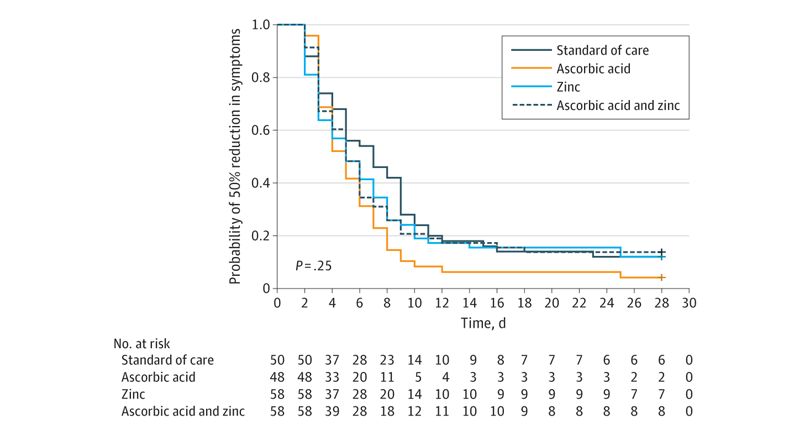
There was no significant difference in the primary outcome of days required to reach a 50% reduction in symptoms among the 4 study groups.
In terms of secondary endpoints, there were no significant differences in any parameter. Few reported any adverse effects, with slightly more in the vitamin C group.
Not promising but also not harmful
This was a prospective trial of two supplements intended to simulate what someone might do if told they had a COVID-19 infection – take large doses for the duration of the illness with the hope that it will support recovery and reduce the risk of severe disease. This trial showed that these products, in these doses, had no measurable effects on the severity of COVID-19 infections. This is perhaps not surprising given the evidence for vitamin C and zinc against colds is not impressive, so there was weak plausibility for a strong treatment effect. However, both of these supplements were generally well tolerated, and there were also no serious negative consequences from their use. So while there is no evidence, based on this trial, to support their use, those that choose to take these supplements in line with this trial can also be reassured that they are unlikely to cause themselves any harm.

